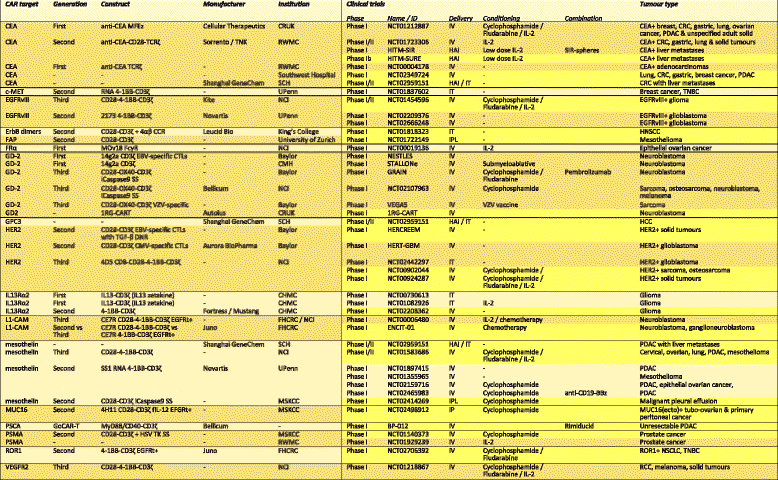
- Table updated and amended from [223]. Clinical trials highlighted in yellow are currently recruiting or not yet recruiting patients as of 28th August 2017 on clinicaltrials.gov. CCR chimeric costimulatory receptor, CD19t truncated CD19, CEA carcinoembryonic antigen, CHMC City of Hope Medical Centre, CMV cytomegalovirus, c-MET c-mesenchymal-epithelial transition, CMH Children’s Mercy Hospital, CNS central nervous system, CRT chemoradiotherapy, CRUK Cancer Research UK, CTL cytotoxic T-lymphocyte, DNR dominant negative receptor, EBV Epstein Barr virus, EGFRt truncated EGFR, EGFRvIII epidermal growth factor receptor variant III, FAP fibroblast activation protein, FHCRC Fred Hutchinson Cancer Research Centre, fIL-12 feline interleukin-12, FRα folate receptor alpha, GR glucocorticoid receptor, HAI hepatic arterial infusion, HCC hepatocellular carcinoma, HER2 human epidermal growth factor receptor 2, HNSCC head and neck squamous cell carcinoma, HSV herpes simplex virus, HyTK hygromycin phosphotransferase-HSV thymidine kinase, IL-2 interleukin-2, IL-13 interleukin-13, IP intraperitoneal, IPL intrapleural, IT intratumoural, IV intravenous, MSKCC Memorial Sloan-Kettering Cancer Centre, NCI National Cancer Institute, NSCLC non-small-cell lung cancer, PDAC pancreatic ductal adenocarcinoma, RCC renal cell carcinoma, RWM Roger Williams Medical Centre, SCH Shanghai Cancer Hospital, SS safety switch, TCR T-cell receptor, TK thymidine kinase, TNBC triple negative breast cancer, UPenn University of Pennsylvania

