Fig. 1
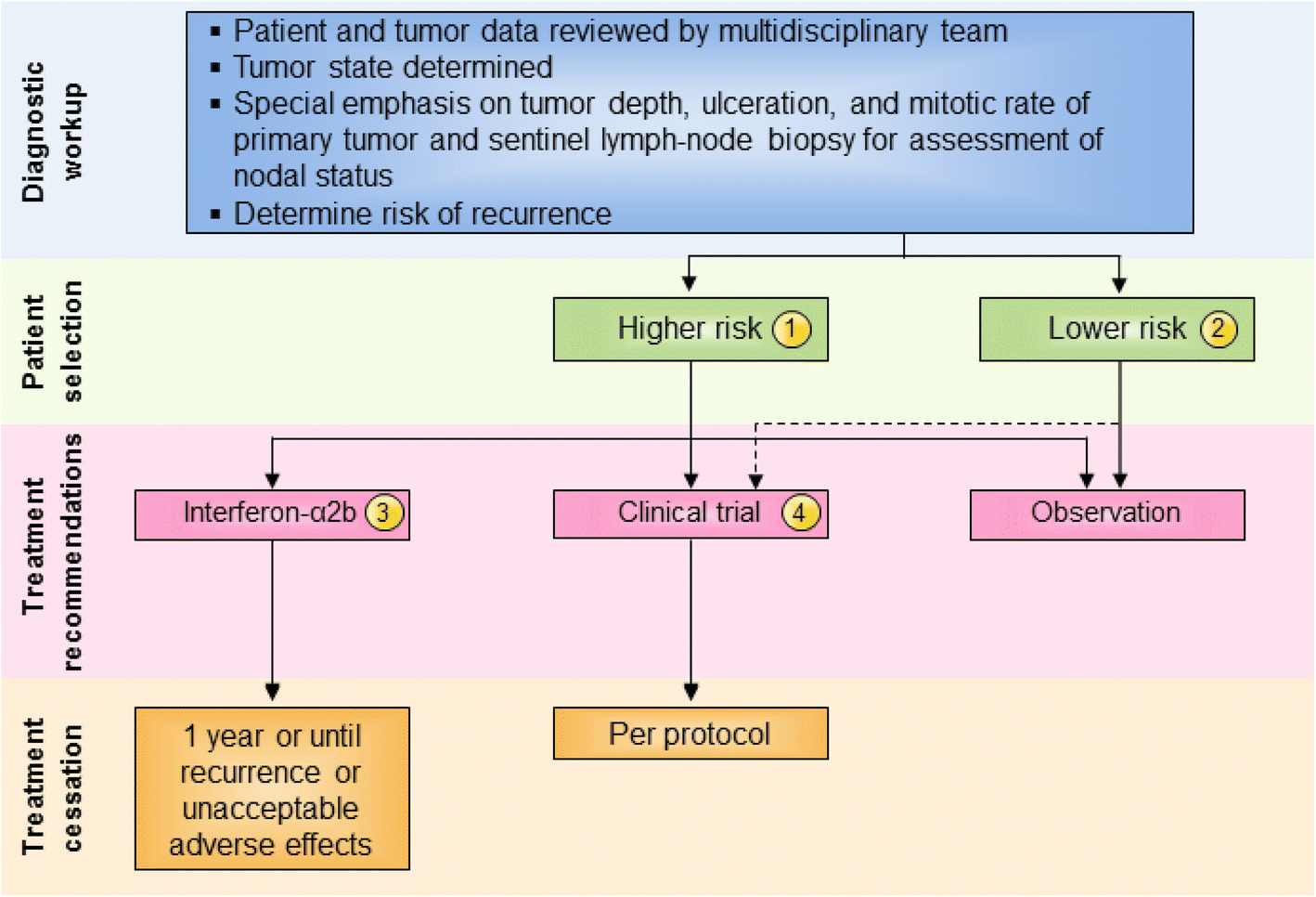
Stage II melanoma immunotherapy treatment algorithm. All treatment options shown may be appropriate, and final selection of therapy should be individualized based on patient eligibility and treatment availability at the physician’s discretion. These algorithms represent consensus sequencing suggestions by the panel. (1) High-risk disease is defined as tumors > 4 mm in depth (with or without ulceration) or > 2–4 mm with ulceration. There is limited consensus on adjuvant therapy for this group with 10% of the panel recommending interferon-α2b, 20% recommending observation, 45 and 15% recommending therapeutic and/or biomarker-based clinical trial participation, respectively, and no panelists recommending pegylated-interferon-α2. (2) There is no evidence that immunotherapy is useful in patients with lower risk stage II melanoma, although the panel did recommend clinical trial participation, if available. Protocol-specific eligibility would need to be followed to select appropriate study candidates. (3) Patients should have a good performance status without evidence of significant depression, psychiatric history or underlying autoimmune disease to be considered for interferon-α2b. There are limited data available on interferon-α2b as treatment for stage II disease. (4) Clinical trials were the preferred treatment recommendation for patients with stage II disease associated with higher risk of tumor recurrence